Kathryn R Napier 1, Megan Tones 2, Chloe Simons 3, Helen Heussler 4, Adam A Hunter 1, Meagan Cross 3, Matthew I Bellgard 5
- 1 Centre for Comparative Genomics, Murdoch University, Perth, WA, 6150, Australia.
- 2 Mater Research, Centre for Children’s Health Research, South Brisbane, QLD, 4101, Australia.
- 3 Foundation for Angelman Syndrome Therapeutics Australia, Salisbury, QLD, 4107, Australia.
- 4 Mater Research, University of Queensland, Children’s Health Queensland Hospital and Health Service, Brisbane, QLD, 4101, Australia.
- 5 Centre for Comparative Genomics, Murdoch University, Perth, WA, 6150, Australia. mbellgard@ccg.murdoch.edu.au.
Abstract
Angelman syndrome (AS) is a rare neurodevelopmental disorder that is characterised by severe global developmental delays, ataxia, loss of speech, epilepsy, sleep disorders, and a happy disposition. There is currently no cure for AS, though several pharmaceutical companies are anticipating drug trials for new therapies to treat AS. The Foundation for Angelman Therapeutics (FAST) Australia therefore identified a need for a global AS patient registry to identify patients for recruitment for clinical trials.The Global AS Registry was deployed in September 2016 utilising the Rare Disease Registry Framework, an open-source tool that enables the efficient creation and management of patient registries. The Global AS Registry is web-based and allows parents and guardians worldwide to register, provide informed consent, and enter data on individuals with AS. 286 patients have registered in the first 8 months since deployment.We demonstrate the successful deployment of the first patient-driven global registry for AS. The data generated from the Global AS Registry will be crucial in identifying patients suitable for clinical trials and in informing research that will identify treatments for AS, and ultimately improve the lives of individuals and their families living with AS.
Keywords:
Angelman syndrome; Disease registry; Global; Interoperable; Open source; Patient reported; Rare disease; Registry framework.
Conflict of interest statement
Ethics approval and consent to participate
The Mater Health Services Human Research Ethics Committee (HREC/13/MHS/76), the Mater Human Research Governance Committee (RG-16-078-AM01), and the Murdoch University Human Research Ethics Committee (2016/216) reviewed and approved this study. Parents and guardians provide informed consent to enter data in the registry on behalf of individuals with AS.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
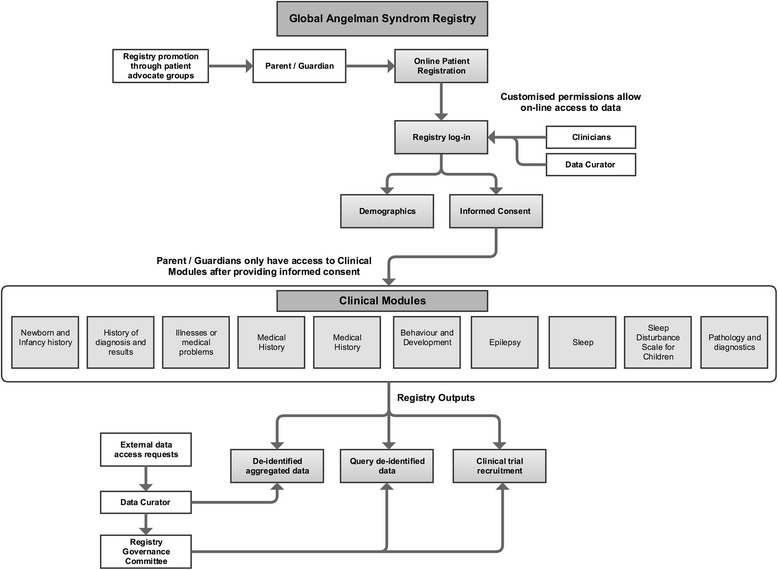
Fig. 1 Functions of the Global Angelman Syndrome Registry. The formatting of this figure is adapted from Napier et al. [18]